Empowering Local Decisions with Isotope Hydrology: A New Frontier for India’s Water Strategy
Isotope hydrology has made tremendous strides in addressing “big hydrology” questions and has revolutionised our understanding of the water cycle by using stable and radioactive isotopes to trace the origin, age, and movement of water. It has improved our understanding of the Indian monsoon and allowed scientists to identify recharge zones, the residence time of groundwater and quantify groundwater-surface water interactions. In fact, the government of India has since launched a major effort to develop new isotope methodologies and monitor isotopic fingerprints of major water sources in spatial and temporal domains through various
departments and R&D centers.
India faces a complex water crisis marked by seasonal scarcity, overexploited groundwater, polluted surface water, and growing demand across sectors, all exacerbated by climate change. These challenges are underpinned by lack of scientific data and efficient technological innovations. It is in this respect; Isotope hydrology can play a more direct and effective role in water resources management. There have been some studies, mainly region-specific and issue-based, but not enough to address the scale of India’s water challenges. These research studies often end up as peer reviewed publications, but have the tremendous potential to provide solutions to pertinent water issues through providing deeper insights into the causes of the problem. With the advent of new-age instruments, the throughput and accuracy of isotope measurements has drastically improved with reduced costs, allowing isotope hydrology readily accessible for water resource management and research.
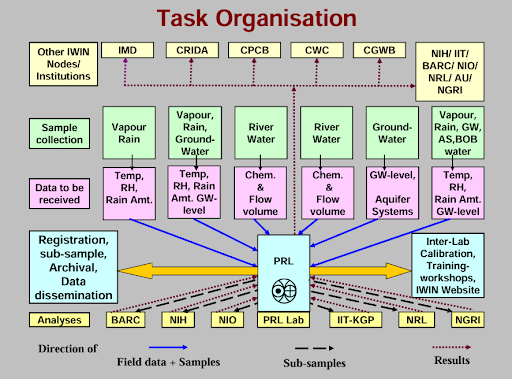
Isotope fingerprinting of waters of India national programme. Source: IWIN
There is a need to identify questions that could be answered by isotope hydrology where the knowledge could inform specific decisions at the local level (ULB, Panchayat). Below are some questions that could be tackled through isotope
Techniques.
Impact of fertilisers and inadequate sanitation on drinking water sources
Multiple studies have shown nitrate in drinking water supplies is particularly high in rural areas, where average levels have been reported to range from 46 mg/L to 100 mg/L (Ward et al., 2018). CGWB (2023) data suggests that about 56% of India’s districts have excessive nitrates defined as more than 45 mg/L in their groundwater. This widespread contamination poses significant health impacts. For instance, a recent World Bank study showed that continuous exposure to nitrate levels exceeding ~50 mg/l in early childhood can lead to “decreased height as an adult, a well-known indicator of overall health and productivity in adulthood” (Zaveri et al., 2020).
The primary sources of nitrate contamination in India’s groundwater are:
- Agricultural runoff: Excessive use of nitrogen-based fertilisers leads to leaching of nitrates into the groundwater.
- Improper human/animal waste disposal: Inadequate management of animal waste and sewage contributes to nitrate levels in water sources.
- Urbanisation: Rapid urban development without proper solid waste management systems exacerbates the problem.
Isotope studies can discriminate between sources of nitrate contamination in groundwater by analyzing the isotopic composition of nitrogen and oxygen atoms within the nitrate (δ15N-NO3>-) molecule. For instance, high δ15N (e.g. >10%) traces a sewage/manure source whereas low δ15N (e.g.<5%) points to a synthetic fertilizer. Although the studies in the global North point to fertilizer as the primary source of nitrate, studies from the global South are more varied. Attribution studies can help to establish whether efforts should focus on sanitation or a gradual shift towards more agro-ecological agricultural practices. Understanding the causal pathways is important, because nitrate is challenging and expensive to remove from well water, once contaminated.
Groundwater quantity and quality issues in canal command areas
Canals are built for surface irrigation yet the seepage from unlined or poorly maintained canals can significantly recharge underlying aquifers and provide a crucial back-up water source, especially in the dry season. Many canal command areas are seeing a sharp increase in “conjunctive use” (use of both canal and groundwater). This is helpful because access to groundwater helps farmers irrigate their crops even after the canal flows stop, allowing them to grow higher value crops. Accurately estimating recharge from canals is essential for building robust water budgets at the distributary level by “water user cooperatives (WUCs)”.
Increasingly governments are promoting “pipe to the field” projects and canal lining to improve efficiency. At the same time, they are promoting crop diversification that requires reliable irrigation.Thus, it becomes doubly important to accurately assess water availability at the distributary level. So, an accurate estimation of recharge from canals is essential for building robust water budgets.
Rainfall and canal water often have distinct isotopic signatures (in δ18O and δ2H values), especially if canal water originates from a distant reservoir or river (Keesari et al., 2017). Groundwater that recharges from canal seepage retains the isotopic fingerprint of the canal water whereas recharge from rainfall reflects local precipitation isotopes. By sampling and comparing isotopic compositions of
canal water, rainfall, and local groundwater, researchers can use mixing models to estimate the proportional contribution of each recharge source.
Many canal command areas in India are beginning to experience soil and groundwater salinity. For instance, salinity in the Tungabhadra Left Bank Canal (TLBC) region, in Karnataka’s Raichur and Koppal districts, has emerged as a significant agricultural concern. There are many reports on soil and water salinity in Indira Gandhi Nahar Project (IGNP), western India and other places (Tewari et al. 1997, Sinha and Navada 2008). This issue ostensibly stems from prolonged irrigation without adequate drainage, leading to waterlogging and the accumulation of salts in the soil. If the causal pathway is established with the support of isotopic data, it could be part of a communications campaign to persuade farmers and irrigation departments in newer canal command areas to avoid getting trapped into an intensive paddy cultivation system right from the beginning.
Identifying the source of contamination in surface water bodies
Phosphate and organic contamination in water bodies leads to excessive algal growth (eutrophication), which depletes oxygen, harms aquatic life, and degrades water quality. Many lakes in India are hypereutrophic. With Jal Jeevan Mission,
and piped water to every home, there is likely to be an increase in greywater flows as well. It is likely that many water bodies will see higher domestic wastewater inflows containing phosphates from detergents. Raw sewage flows are also increasing
as people switch to flush toilets. Further agricultural runoff, increasingly laden with fertilizers is also contributing to deterioration of surface water bodies.
Once polluted, these water sources will be lost to the community for livelihood and domestic in-situ uses. This is especially important to prevent eutrophication of existing drinking water reservoirs. Water may need to be imported from distant sources at considerable cost, even spurring conflicts over the resource.
Different sources (e.g., fertilizers, manure, detergents) often have characteristic phosphate (δ18O), nitrate (δ15N, δ18O) or sulphate (δ34S) values and can be used to trace whether contamination is from sewage, fertilizer, or industrial sources, helping link surface water bodies to pollution sources (Pillai et al., 2024, Keesari 2023, IAEA 2013). Again, source apportionment studies on these nutrient sources can help inform management. For instance, the specific composition of nutrients will determine the course of action – whether catchment level management on non-point source pollution is needed or investments in sewerage and STPs would be useful.
Factors leading to salinisation of freshwater coastal aquifers
There is evidence that indicates increasing salinity in coastal aquifers across India – primarily due to seawater intrusion, exacerbated by over-extraction of groundwater, climate change, and sea-level rise. In deltaic areas, changing river discharge regimes
as consequence of human interventions and climate change, which amplifies seasonality and inter-annual variations, are also driving intrusion along distributaries, particularly in the East coast. Approximately 7% of India’s coastal areas are affected by seawater intrusion, with some regions experiencing intrusion up to 14 km inland.
But many of these coastal regions (such as the Cauvery Delta) have practiced intensive irrigation for decades, even centuries. It would be useful to distinguish the source of salinity among seawater intrusion, irrigation, modern agricultural chemicals or past (ancient) marine transgressions.
A range of isotopes can help detect the causes of salinity in coastal aquifers by distinguishing between different sources and processes that contribute to salinization. For instance, stable isotope ratios of B, S and Cl have been used to distinguish between seawater intrusion and anthropogenic sources like fertilizers or wastewater.Stable water isotopes of O and H (δ18O and δ2H) along with environmental tritium (3H) can be used to identify whether saline water is from modern seawater intrusion, evaporated irrigation return flows, or paleowaters as seawater has a distinct isotopic signature compared to local meteoric water (Rao et al. 1998).
Urban hydrology and groundwater balance
City water balances are important because they provide a comprehensive picture of how water enters, moves through, and exits an urban system, enabling informed decisions on sustainable water management. Traditionally city water balances have
tended to include piped supply and demand. They are used to highlight mismatches between available water and demand (domestic, industrial, institutional) and to plan new infrastructure investments or retrofits.
In recent years, it is becoming common to develop water balances that link water resources and water supply to inform integrated urban water management (Kulranjan et al., 2023). These connect surface water, groundwater, wastewater, and stormwater, encouraging cities to shift from siloed approaches to circular urban water systems. Groundwater, however, tends to be a “black box”, as both recharge and extraction are poorly quantified. Isotope techniques can help differentiate recharge from rainfall, lakes, and leaking water supply systems (Brauns et al., 2022). Artificial radioisotopes are also being employed to quantify the groundwater recharge from rain (Rangarajan and Athavale 2000), which can help in preparing the water budget models and recommend effective plans for sustainable development of groundwater resources.
Promoting spring-shed management in Himalayan states
An important contribution of isotope hydrology is to spring protection, which requires identification of source areas and timing of recharge to springs (e.g., intake of a drinking water well). Springs are the main source of supply for many hill communities. As quality deteriorates, water treatment becomes more expensive. Springs in valleys often derive from higher elevation recharge (Sharma and Mandal, 1999).
The altitude effect on stable isotope ratios of O and H in precipitation refers to how the stable isotope composition of precipitation—and therefore groundwater recharge—systematically changes with elevation. This is a fundamental concept in isotope hydrology that can help identify recharge zones and track water movement (Mook, 2000). Tracing the origins of spring discharges is valuable as it averts polluting industries or sewage contamination in recharge zones of springs supplying large populations.
The Bhabha Atomic Research Centre (BARC), under the Department of Atomic Energy (DAE), has conducted extensive research on spring rejuvenation employing advanced isotope techniques. These studies have successfully demonstrated the critical role and applicability of isotope technology in understanding the hydrodynamics of spring sheds, assessing recharge mechanisms, and guiding effective management strategies. The insights gained from these investigations provide robust scientific evidence that can significantly enhance the sustainable management of spring ecosystems. It is imperative that these scientific findings be integrated into water resource policy frameworks and decision-making processes to ensure the long-term preservation and rejuvenation of springs, which are vital freshwater sources for many communities (Keesari et al., 2025).
Maximizing the benefits of isotope technology through upscaling Grounded isotope hydrology requires local field-based studies. Importantly, many empirical studies are needed to discern patterns and perform meaningful meta-analyses. The Government of India has prepared a very accessible handbook on interpretation of stable isotopes and the Oasis-G Tool. Although it focuses mainly on rainfall and recharge (δ18O and δ2H values) similar handbooks may be prepared for other common isotopic tracers.
A strong case might then be made to train post-graduates and research scholars in these isotope techniques with the object of answering more directly and locally water resources management problems. This will require starting with a “user validated” research question, then rigorously designing a simple study to answer the question. The study would entail carefully designed local sampling, designed to answer a management question at the appropriate level. Ideally, the study would be done in partnership with the decision-making agency.
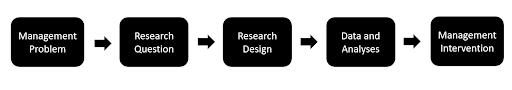
Approach to groundwater isotope hydrology
To answer these questions well, there is a need for strong, robust research design (sampling methodology, sample collection protocols, timing and frequency of sample collection etc.). Hypothesis testing, which is central to grounded stable-isotope hydrology, is also a good way of developing research capacity in students of the scientific process while also addressing critical societal problems.
This article was originally published in the Bhabha Atomic Research Centre Newsletter—Harnessing Isotopes for Smarter Water Management (March-April 2025). Access the full newsletter here.
Authors Veena Srinivasan, Richard Taylor
Originally published in the Bhabha Atomic Research Centre Newsletter—Harnessing Isotopes for Smarter Water Management. (March-April 2025)
Follow us to stay updated about our work.




